pH Probe Calibrations and Maintenance
The success or failure of pH measurement depends on the proper application of the probe and proper subsequent maintenance of the probe.
The procedures described within apply to the most common pH probe in use today and that is the flat surface combination pH probe.
The most common failure mode associated with pH probes is breakage. The pH electrode is a very thin glass membrane that is easily damaged.
Foreign object damage within the installation or mechanical shock during calibration are often the culprits. The next most common cause of
failure is a plugged reference junction. The reference junction consists of a porous material, usually ceramic or Teflon, that must remain open.
The junction creates a fluid interface between the reference material which is a liquid and the process fluid. A flow, albeit infinitesimally low, must exist from the reference electrode to the process fluid. In environments where there are high solids, oils or grease this junction can become plugged.
pH Probe Cleaning
 When the reference junction becomes plugged the probe will become sluggish and unresponsive.
In many cases the junction can be cleaned with aggressive alkaline cleaners (for oil plugging)
or dilute acids (for salt deposits) or a combination of both. In most cases, for probes with large
junction surface areas the junction material can be scraped away with a screw driver revealing a new surface.
Aggressive procedures are sometimes necessary to bring life back to a dead probe.
The glass pH sensing membrane may also require service in some applications. This membrane can become dehydrated
or coated with a thin layer of deposits. The best procedure for cleaning or rehydrating the glass is to soak the
probe in a pH buffer of 4.0 for several hours. If this does not work then immersing the probe in hot buffer 4.0 solution will usually work.
When the reference junction becomes plugged the probe will become sluggish and unresponsive.
In many cases the junction can be cleaned with aggressive alkaline cleaners (for oil plugging)
or dilute acids (for salt deposits) or a combination of both. In most cases, for probes with large
junction surface areas the junction material can be scraped away with a screw driver revealing a new surface.
Aggressive procedures are sometimes necessary to bring life back to a dead probe.
The glass pH sensing membrane may also require service in some applications. This membrane can become dehydrated
or coated with a thin layer of deposits. The best procedure for cleaning or rehydrating the glass is to soak the
probe in a pH buffer of 4.0 for several hours. If this does not work then immersing the probe in hot buffer 4.0 solution will usually work.
 In most cases the aggressive procedures described above will not be necessary.
Cleaning can usually consist of simply wiping the surface of the probe with a cloth or rag.
Care must be taken not to break the glass membrane. The glass is thin and delicate but strong enough to handle aggressive cleaning with a cloth.
In most cases the aggressive procedures described above will not be necessary.
Cleaning can usually consist of simply wiping the surface of the probe with a cloth or rag.
Care must be taken not to break the glass membrane. The glass is thin and delicate but strong enough to handle aggressive cleaning with a cloth.
pH Probes will likely be the most service intensive device anywhere within a plant. Most measuring elements such as flow sensors,
temperature sensors, etc. rarely need service. However, pH probes, in some installations will require service as often as 2 or 3 times a day.
For most applications, however, cleaning and calibrating once monthly will suffice.
Unlike flow sensors, temperature sensors, or just about any other type of sensor that we commonly use in industry, the pH electrode is relatively
unstable and maintenance intensive. pH Probes will normally require weekly or monthly cleanings and monthly calibrations. The actual frequency
is a function of the installation environment but could be as often as a couple of times a day.
pH Probe Calibration
pH Electrodes must be calibrated using a two point method with the appropriate buffer standards. There are some manufacturers which describe
single point calibrations, however we cannot endorse this method. pH probe failure modes are numerous and can escape conventional single point checks.
Therefore a two point calibration must be performed.
To perform a two point calibration two buffer solutions are required, as the method implies. These buffers should be based upon the normal measurement range that probe operates in. An effluent monitoring probe or neutralization probe should use pH buffers 7.0 and 10.0. Likewise, a pH probe in a hexavalent chromium reduction system, for example, should be calibrated with a 2.0 (or 4.0) and a 7.0 buffer solution.
Care must be taken not to cross contaminate the buffers. As the name implies pH buffers solutions are designed to be buffering
at their advertised pH value. They can, however, become easily contaminated which, of course, renders them useless.
The procedures that follow on the next page indicate that the probe must be rinsed and wiped dry when moving from one buffer to the next.
As one might expect it is easy to drag out buffer from one beaker to the next. Therefore rinsing the probe and wiping dry is essential
when moving between buffer solutions. DI or demineralized water is not required to rinse the probes, any clean water source will suffice.
On another topic, for a moment, it is important to point out that DI or demineralized water should not
be used to store a probe as this will shorten probe life. A pH probe should be stored in a solution
with high ionic strength, preferably a pH buffer solution of 4.0.
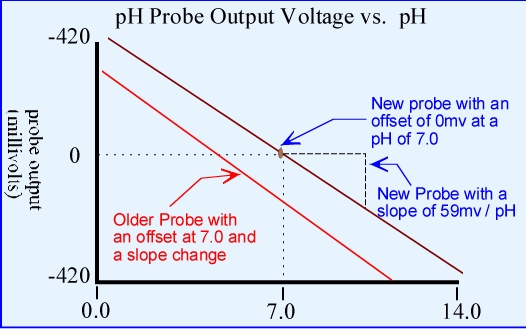 A new pH probe will have an offset of 0 mv at a pH of 7.0 and a response
of 59mv per pH unit change (slope).
A new pH probe will have an offset of 0 mv at a pH of 7.0 and a response
of 59mv per pH unit change (slope).
As a pH probe ages both the slope and
offset of the probe will change. This is what dictates probe calibration frequency.
Additionally the response time of the probe will change.
Generally speaking, when an offset of more than 30 mv (at 7.0 pH) develops or more than
2 minutes is required for a probe to stabilize in a buffer solution a probe has reached end of life or needs reconditioning.
